View Orchid ID Profile
Primary Authorship
Rivers-Auty, J., Bond, A.L., Grant, M.L., and Lavers, J.L. (2023). The one-two punch of plastic exposure: Macro-and micro-plastics induce multi-organ damage in seabirds. Journal of Hazardous Materials 442, 130117.
Link – Journal of Hazardous Materials
PDF – Rivers-Auty et al 2023
Mylius, K.A., Lavers, J.L., Woehler, E.J., Rodemann, T., Keys, B.C., and Rivers-Auty, J. (2022). Foraging strategy influences the quantity of ingested micro-and nanoplastics in shorebirds. Environmental Pollution, 120844.
Link – Pollution (Journal)
PDF –Mylius, Rivers-Auty 2022
Rivers-Auty J, Tapia VS, White CS, et al., 2021. Zinc Status Alters Alzheimer’s Disease Progression through NLRP3-Dependent Inflammation. Journal of Neuroscience, Accepted, to be published.
Link– Journal of Neuroscience
PDF–Rivers-Auty 2021 Journal of Neuroscience – preprint
Rivers-Auty J, Mather AE, Peters R, Lawrence CB, Brough D, (2020) Anti-inflammatories in Alzheimer’s disease—potential therapy or spurious correlate? Brain Communications 2020; 2,
Link– Brain Communications
PDF–Rivers-Auty 2020 Brain Communications
Rivers-Auty, J., Daniels, M. J. D., Colliver, I., Robertson, D. L. & Brough, D. Redefining the ancestral origins of the interleukin-1 superfamily. Nat. Commun. 1156, doi:10.1038/s41467-018-03362-1 (2018).
Link –Nature Communications –
Pubmed https://www.ncbi.nlm.nih.gov/pubmed/29559685
PDF –Rivers-Auty et al. 2018 Nat. Comms
White CS, Lawrence CB, Brough D, Rivers-Auty J (2016) Inflammasomes as therapeutic targets for Alzheimer’s disease. Brain Pathology: doi:10.1111/bpa.12478
Link –Brain Pathology
PDF –White, et al. RWhite et al. Rivers-Auty 2016 Inflammasomes in Alzheimer’s disease
Daniels MJD*, Rivers-Auty J*, Schilling T, Spencer NG, Watremez W, Fasolino V, Booth SJ, White CS, Baldwin AG, Freeman S, Wong R, Latta C, Yu S, Jackson J, Fischer N, Koziel V, Pillot T, Bagnall J, Allan SM, Paszek P, Galea J, Harte MK, Eder C, Lawrence CB, Brough D (2016) Fenamate NSAIDs inhibit the NLRP3 inflammasome and protect against Alzheimer/’s disease in rodent models. Nature Communications 7.
doi:10.1038/ncomms12504 *equal contributors
Link –Nature Communications
PDF –Daniels, Rivers-Auty, Brough 2016- Nature Communications
Rivers-Auty J, Brough D, 2015. Potassium efflux fires the canon: Potassium efflux as a common trigger for canonical and noncanonical NLRP3 pathways. European Journal of Immunology. 45: 2758-61.
Link –European Journal of Immunology
PDF –Rivers-Auty and Brough 2015-European_Journal_of_Immunology
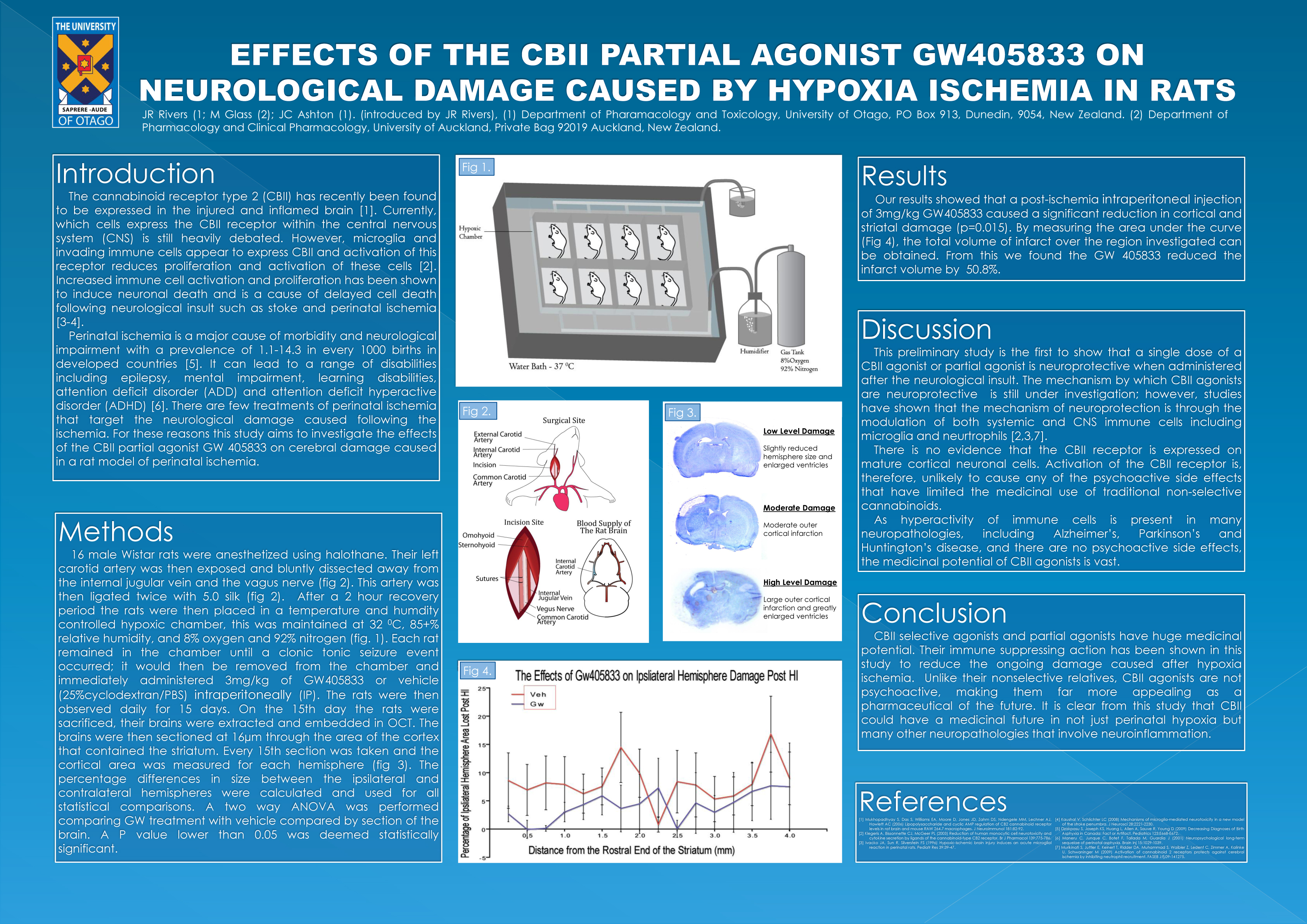
Rivers-Auty J, Smith PF, Ashton JC, 2014. The cannabinoid CB2 receptor agonist GW405833 does not ameliorate brain damage induced by hypoxia-ischemia in rats. Neuroscience Letters. 569, 104-9.
Link –http://www.ncbi.nlm.nih.gov/pubmed/24721671
PDF –Rivers-Auty 2014 -The cannabinoid CB2receptor agonist GW405833 does not amelioratebrain damage induced by hypoxia-ischemia in rats
Rivers-Auty J. An evolutionary perspective on the immunomodulatory role of hydrogen sulphide. Medical Hypotheses; 85: 612-7.
Link –http://www.sciencedirect.com/
PDF Rivers-Auty J An evolutionary perspective on the immunomodulatory role of hydrogen sulphide- Medical Hypotheses
Rivers-Auty J. 2014. The blind leading the blind: animal facility staff and researchers working together to reduce bias in animal research. Royal Society of New Zealand ANZCCART.
Link – http://anzccart.org.nz/wp-content/uploads/2013/12/Mixing-it-up-ANZCCART-2014.pdf
Rivers-Auty J, Ashton JC. 2013. Neuroinflammation in Ischemic Brain Injury as an Adaptive Process. Medical Hypotheses; 82(2): 151-8.
Link –http://www.ncbi.nlm.nih.gov/pubmed/24345344
PDF –Rivers-Auty 2013 Neuroinflammation in Ischemic Brain Injury as an Adaptive Process
Rivers-Auty J, Ashton JC. 2013. Vehicles for Lipophilic Drugs: Implications for Experimental Design, Neuroprotection, and Drug Discovery. Current Neurovascular Research. 10(4);356-60.
Link –http://www.ncbi.nlm.nih.gov/pubmed/23937198
PDF- Rivers-Auty 2013 Vehicles for Lipophilic Drugs Implications for Experimental Design Neuroprotection and Drug Discovery
Rivers-Auty, J., and M. Bhatia. 2013. Hydrogen sulfide, systemic inflammatory response syndrome (SIRS) and compensatory anti-inflammatory response syndrome (CARS) following sepsis. OA Inflammation. 1(1):2-10.
Link –https://www.oapublishinglondon.com/article/413
PDF-Rivers-Auty 2013 Hydrogen sulfide, systemic inflammatory response syndrome (SIRS) and compensatory anti-inflammatory response syndrome (CARS) following sepsis.
Rivers, J.R., and J.C. Ashton. 2013. Age matching animal models to humans – theoretical considerations. Current Drug Discovery Technologies. 10(3):177-81
Link –http://www.ncbi.nlm.nih.gov/pubmed/23363234
PDF-Rivers 2013 Age Matching Animal Models to Humans – Theoretical Considerations
Rivers, J.R., A. Badiei, and M. Bhatia. 2012. Hydrogen sulfide as a therapeutic target for inflammation. Expert Opinion on Therapeutic Targets. 16:439-449.
Link –http://www.ncbi.nlm.nih.gov/pubmed/22448627
PDF-Rivers 2012 Hydrogen sulfide as a therapeutic target for inflammation
Rivers, J.R., S.D. Maggo, and J.C. Ashton. 2012. Neuroprotective effect of hydroxypropyl-beta-cyclodextrin in hypoxia-ischemia. NeuroReport. 23:134-138.
Link –http://www.ncbi.nlm.nih.gov/pubmed/22182974
PDF-Rivers 2011 Neuroprotective effect of hydroxypropyl-b-cyclodextrin in hypoxia ischemia
Rivers, J.R., B.A. Sutherland, and J.C. Ashton. 2011. Characterization of a rat hypoxia-ischemia model where duration of hypoxia is determined by seizure activity. Journal of Neuroscience Methods. 197:92-96.
Link –http://www.ncbi.nlm.nih.gov/pubmed/21320527
PDF-Rivers 2011 Characterization of a rat hypoxia-ischemia model where duration of hypoxia is determined by seizure activity
Rivers, J.R., and J.C. Ashton. 2010. The development of cannabinoid CBII receptor agonists for the treatment of central neuropathies. Central Nervous System Agents for Medicinal Chemistry. 10:47-64.
Link –http://www.ncbi.nlm.nih.gov/pubmed/20236042
PDF-Rivers 2010 The Development of Cannabinoid CBII Receptor Agonists for the Treatment
Middle Authorship
Hadjidemetriou M, Rivers-Auty J, Papafilippou L, Eales J, B KKA, Lawrence CB, Kostarelos K (2021) Nanoparticle-enabled enrichment of longitudinal blood proteomic fingerprints in Alzheimer’s disease. ACS nano:Accepted to be published
Link -Coming
PDF- Coming
Haley MJ, White CS, Roberts D, O’Toole K, Cunningham CJ, Rivers-Auty J, O’Boyle C, Lane C, Heaney O, Allan SM, Lawrence CB (2019) Stroke Induces Prolonged Changes in Lipid Metabolism, the Liver and Body Composition in Mice. Transl Stroke Res. doi:10.1007/s12975-019-00763-2
Link – Translation Stroke Research
PDF – Haley et al. 2020
Tapia VS, Daniels MJD, Palazon-Riquelme P, Dewhurst M, Luheshi NM, Rivers-Auty J, Green J, Redondo-Castro E, Kaldis P, Lopez-Castejon G, Brough D (2019) The three cytokines IL-1beta, IL-18, and IL-1alpha share related but distinct secretory routes. J Biol Chem.
doi:10.1074/jbc.RA119.008009
Link – Journal of Biological Chemistry
PDF- Tapia et al. JBC
Crilly S, Njegic A, Laurie S, Fotiou E, Hudson G, Barrington J, Webb K, Young H, Badrock A, Hurlstone A, Rivers-Auty J, Parry-Jones A, Allan S, Kasher P (2018) Using zebrafish larval models to study brain injury, locomotor and neuroinflammatory outcomes following intracerebral haemorrhage. F1000Research 7 (1617). doi:10.12688/f1000research.16473.1
Link –F1000
PDF- Crilly et al F1000 2018
Hoyle C, Rivers-Auty J, Lemarchand E, Vranic S, Wang E, Buggio M, Rothwell N, Allan S, Kostarelos K, Brough D (2018) Small, Thin Graphene Oxide Is Anti-Inflammatory Activating Nuclear Factor Erythroid 2-Related Factor 2 (NRF2) Via Metabolic Reprogramming. ACS Nano. DOI: 10.1021/acsnano.8b03642
Link –ASC Nano
PDF- Hoyle et al ASC Nano
Rajkovic I, Wong R, Lemarchand E, Rivers-Auty J, Rajkovic O, Garlan2018 Hoyle et al ASC Nanoda C, Allan SM, Pinteaux E (2018) Pentraxin 3 promotes long-term cerebral blood flow recovery, angiogenesis, and neuronal survival after stroke. J Mol Med:1-14
Link –J Mol Med
PDF- Rajkovic et al. J Mol Med 2018
Diamond CE, Leong KWK, Vacca M, Rivers-Auty J, Brough D, Mortellaro A (2017) Salmonella typhimurium-induced IL-1 release from primary human monocytes requires NLRP3 and can occur in the absence of pyroptosis. Scientific Reports 7:6861. doi:10.1038/s41598-017-07081-3
Link –Scientific reports
PDF- Diamond Scientific Reports 2017
Xu J, Begley P, Church SJ, Patassini S, McHarg S, Kureishy N, Hollywood KA, Waldvogel H, Liu H, Zhang S, Lin W, Herholz K, Turner C, Synek BJ, Curtis MA, Rivers-Auty J, Lawrence CB, Kellett KAB, Hooper NM, Vardy ERLC, Wu D, Unwin RD, Faull RLM, Dowsey AW, Cooper GJS, (2016). Elevation of brain glucose and polyol-pathway intermediates with accompanying brain-copper deficiency in patients with Alzheimer’s disease: metabolic basis for dementia. Scientific Reports. 6:27524.
Link –Scientific reports
PDF- Xu et al. Scientific Reports 2017
Baldwin AG, Rivers-Auty J, Daniels MJD, White CS, Schwalbe CH, Schilling T, Hammadi H, Jaiyong P, Spencer NG, England H, Luheshi NM, Kadirvel M, Lawrence CB, Rothwell NJ, Harte MK, Bryce RA, Allan SM, Eder C, Freeman S, Brough D (2017) Boron-Based Inhibitors of the NLRP3 Inflammasome. Cell Chemical Biology. 24(11), 1321-1335.
Link –Cell Chemical Biology
PDF-Baldwin et al. 2017 Cell Chemical Biology
Badiei A, Rivers-Auty J, Ang A, Bhatia M. Inhibition of hydrogen sulfide production by gene silencing attenuates inflammatory activity of LPS-activated RAW264.7 cells. Applied Microbiology and Biotechnology 2013;97(17):7845-52.
Link –http://www.ncbi.nlm.nih.gov/pubmed/23838794
PDF-Badiei-2013-Inhibition of hydrogen sulfide production by gene silencing
Ang AD, Rivers-Auty J, Akhil Hegde A, Ishii I, Bhatia M. The effect of CSE gene deletion in caerulein-induced acute pancreatitis in the mouse. American Journal of Physiology – Gastrointestinal and Liver Physiology 2013;305: 712-21.
Link –http://www.ncbi.nlm.nih.gov/pubmed/24008358
PDF-Ang et al AJP 2013 The effect of CSE gene deletion in caerulein-induced acute pancreatitis
Book Chapters
Jupp, L., J.R. Rivers, and M. Bhatia. 2013. Hydrogen sulfide and substance P in acute pancreatitis. In Adaptation Biology and Medicine. Vol. 7. A.R. Popescu and P.K. Singal, editors. Narosa Publishing House, New Delhi.
Link –http://www.amazon.ca/Adaptation-Biology-Medicine-Vol-Developments/dp/toc/8184872143
PDF-Jupp and Rivers-Auty 2013 Hydrogen Sulfide and Substance P in acute Pancreatitis
Rivers, J.R., and J.C. Ashton. 2012. Neonatal asphyxia and stroke: morbidity, models, consequences, and treatments In Hypoxia: Causes, Types and Management. D.Vordermark, editor. Nova Publishers. 108-130.
Link –https://www.novapublishers.com/catalog/product_info.php?products_id=49272
PDF-Rivers 2012 Neonatal asphyxia and stroke morbidity models consequences and treatments