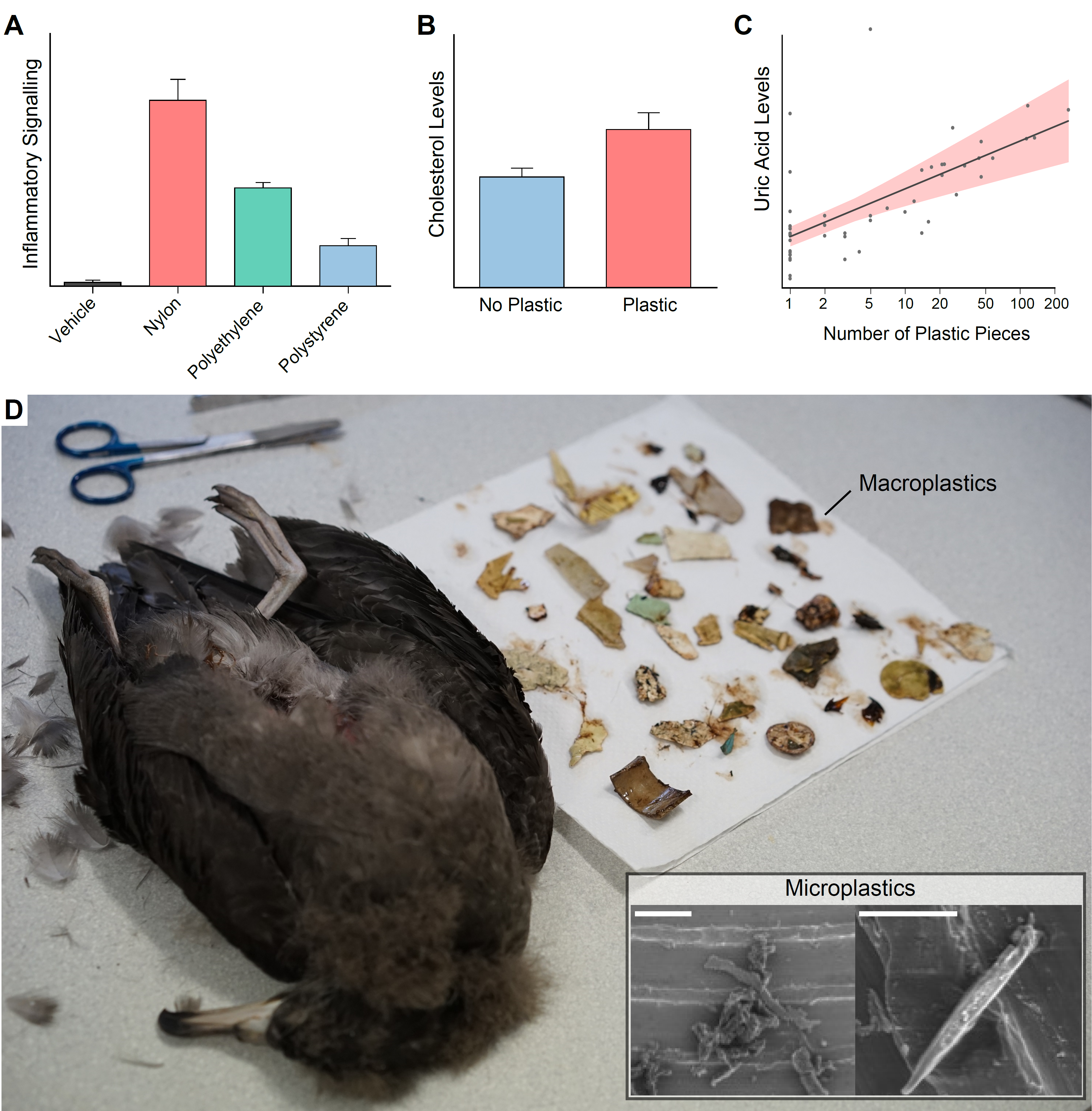
Plastics are inexpensive, durable, lightweight, versatile material composed of long hydrocarbon chains. These properties have led to widespread use of plastics particularly in single-use packaging. Unfortunately, this has caused a global plastic pollution problem with between 5 and 13 million tonnes of plastic entering our oceans annually. Microplastics are particles of submillimetre plastic. Microplastics have particular biological importance as they can enter and sequester in organs and can be taken up by individual cells, where they are having, as yet, unknown health consequences. Primary microplastics are manufactured in the micron-scale such as microfiber clothing, while secondary microplastics are macroplastics that are broken down into micron-scale plastics through exposure to U.V. light, erosion and digestive fragmentation. My research has discovered that microplastic can activate the inflammatory receptors resulting in inflammation. This depends on both the composition of the plastic and the size of the microplastic. However, the health consequences of this are not known. Our research is investigating the health consequences of microplastic exposure in both humans and wildlife. Using cell culture we can investigate the consequences of plastic exposure to human cells and we can also look at pathology that occurs in wildlife that are exposed to high plastic due to their eating habits. These methods help us understand the microplastics problem on a global scale from wildlife to humans.
Email me if you are interested in doing a PhD with me and the wonderful Dr. Jenn Lavers (IMAS, UTAS) and the brilliant Dr. Alex Bond (Natural History Museum, UK), on this topic.
jack.auty@utas.edu.au
Figure. A) In cell culture models microplastics were inflammatory and this response differed between polymer composition. B and C) In seabirds, plastic ingestion was associated with significantly increased cholesterol (B) and uric acid (C) in the plasma. D) Seabirds fatally ingest macroplastics, but the sublethal effects of microplastics are unknown (Scale bars 10µm) (Lavers et al. 2014).